Genome regulation is crucial to implement genomic information and to shape the properties of cells and organisms. Differences in gene expression are likely to specify much of the phenotypic differences between and within species. Cellular function results largely from the dynamic interplay between DNA or RNA and regulatory processes. The genome is regulated at multiple levels, and cells need to integrate external and internal cues and coordinate different regulatory levels to properly exert biological functions. These are exciting times to study the regulation and function of genomes owing to unprecedented breakthroughs in technology and information. Genome-wide approaches such as next-generation sequencing are powerful to understand the information flow from genotype to phenotype, and the regulatory complexity accompanying life and disease.
We use the fission yeast, Schizosaccharomyces pombe, and more recently turquoise killifish, Nothobranchius furzeri, to study the dynamic changes and plasticity of gene expression programs as a function of cell quiescence/dormancy and ageing, and the effect of various genetic and environmental perturbations. We are also interested in genetic diversity, genome evolution, and the complex interactions between genotypes, phenotypes, and the environment. Fission yeast is easy to handle, featuring powerful genetics and a well-defined genome. The relative simplicity of the yeast cell promises a deeply satisfying systems-level understanding of its inner workings within our lifetime.
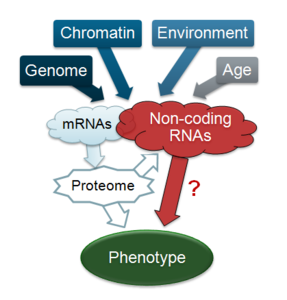
Non-coding RNAs: The transcriptome is dynamically tuned to environmental factors, linking genotype with phenotype. Recent findings indicate that the transcribed portions of genomes are more pervasive than anticipated. Traditionally, coding sequences have enjoyed the main attention of biologists, but the emerging profusion of non-coding RNAs (ncRNAs), which can greatly outnumber coding transcripts, raises important questions about their overall significance and regulation. How much genetic information is transacted by ncRNAs is hotly debated in the field: Do most ncRNAs reflect mere opportunistic transcriptional noise, or does this ‘dark matter’ form a massive hidden network of regulatory information that controls gene expression and mediates gene-environment interactions? Fission yeast provides a potent platform for comprehensive insight into ncRNA function and regulation. Applications of next-generation sequencing, combined with powerful yeast genetics, now create tremendous opportunities to investigate both global contributions and specific roles of ncRNAs in genome regulation and phenotypic variation.
Cellular ageing: Age is the major risk factor for numerous diseases, including cardiovascular, diabetes, neurodegeneration, and cancer. While specific disease processes have long been a focus of biomedical research, there is a growing realisation that fundamental aspects of ageing are an essential part of the problem. Age-associated decline is not invariably fixed, and basic research on ageing is vital to combat the predicted epidemic of age-related diseases. Research into ageing is a young field that has seen immense progress in recent years, galvanised by discoveries that single gene mutations and dietary restriction can extend a healthy lifespan. Remarkably, conserved nutrient-signalling pathways affect longevity from yeast to mammals. How genome regulation in general, and ncRNAs in particular, affect the ageing process is largely unknown. We study how genetic, environmental and regulatory factors affect the chronological lifespan of quiescent yeast cells. To pursue this multi-dimensional research with a holistic perspective, we apply next-generation sequencing, microarrays, proteomics, and functional profiling of mutant strains. Integration of our genomics, genetics, cell biological and biochemical approaches, supported with computational data mining, provides insight into the multi-layered gene expression networks and of regulatory strategies orchestrating biological processes. This research, performed under controlled conditions in a simple model, will support a systems-level understanding of universal relationships between genotype, environment, and phenotype. Our research questions are basic in nature, but they have broad relevance for other organisms and for medical applications.
Gene function: How different genotypes and environments produce complex phenotypes is central for genetics research. Cellular characteristics result largely from the dynamic interplay between DNA or RNA and the regulatory apparatus. The information flow from a given genome (genotype) and environment to the resulting phenotype is mediated by the transcriptome, with unknown overall contribution from numerous non-coding RNAs.
Past and current support of our research: